By Alyx Arnett
For Amy Lopez, watching her son Nick Lopez, 20, who has Down syndrome, deal with obstructive sleep apnea (OSA) has been challenging. Diagnosed at a young age, Nick’s journey with OSA began early due to his Down syndrome-related anatomical challenges, including a larger tongue that frequently blocked his airway.
Despite attempts to manage his condition with firstline treatments—including an adenotonsillectomy and CPAP—Nick’s breathing often required vigilance from Amy, who would stay in the same bed with him. “I’d have to keep a close eye on his breathing. Sometimes he’d stop breathing briefly, and I’d have to readjust him,” says Amy.
Nick started on CPAP in 2017, but his sleep apnea worsened over time. “The pressure got bumped up to 15, and he just couldn’t keep it on,” Amy says. Even with CPAP, Nick’s apnea-hypopnea index (AHI) was close to 20. Since patients with Down syndrome are already predisposed to cardiac issues,1 Amy was concerned that Nick’s sleep apnea—also linked to worsened cardiovascular outcomes—could further put his heart at risk.2
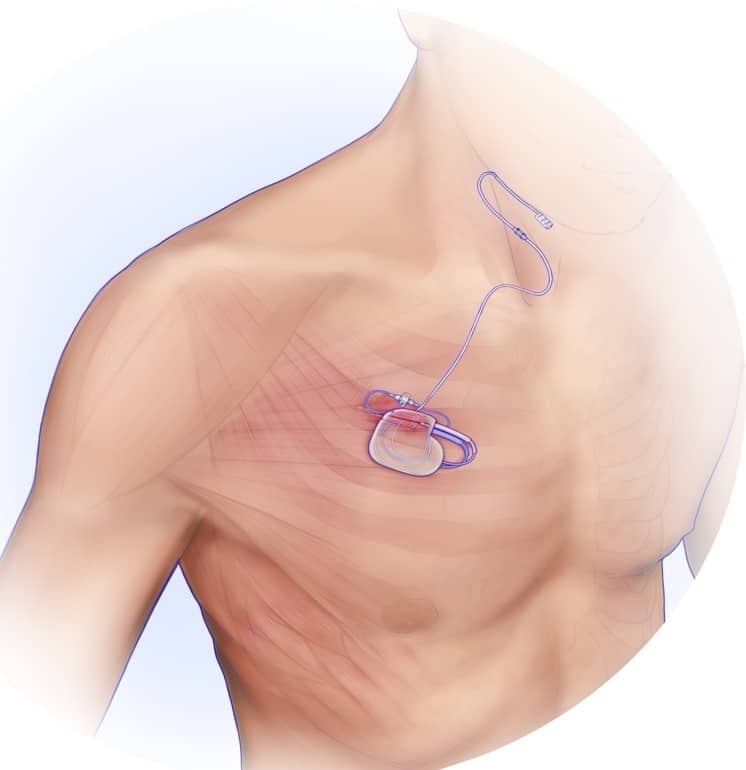
But Amy and her husband Mike refused to give up hope. When Nick’s CPAP pressure became intolerable to him, Amy consulted her father-in-law, a retired internal medicine doctor, William Lopez. It was William who suggested a neurostimulator for OSA, Inspire by Inspire Medical Systems, which received its initial US Food and Drug Administration (FDA) approval in 2014.
Evaluation for Neurostimulation Treatment
At that time, the neurostimulator was FDA-approved only for adults, so Nick, 17, had to wait. Then, with Nick’s grandfather’s research and encouragement and Norman Friedman, MD, an otolaryngologist and director of the Children’s Sleep Medicine Laboratory at Children’s Hospital Colorado, guiding the way, Nick was evaluated and found to be a suitable candidate for the neurostimulator.
A drug-induced sleep endoscopy showed that Nick would likely benefit from the treatment, and his sensory issues—common in patients with Down syndrome—weren’t so severe that they were likely to impede success. “If you can’t go up to the patient and put a cell phone on their chest, which is where the device would be, then they’re not going to be able to be a good candidate because you’re not going to be able to turn it on at night,” Friedman says.
Nick was also a good candidate because, in addition to not tolerating CPAP well, he had a strong family support system, according to Friedman. “Nick’s grandfather is a (retired) physician. He’s familiar with the negative consequences of untreated sleep apnea. The mom is very vested. And Nick is just an amazing, amazing young man that was very motivated with a very good attitude,” he says.
In January 2023, at 19 years old, Nick underwent the surgery. The device was surgically placed under the skin above his chest and connected to the hypoglossal nerve, which controls tongue movement and other key airway muscles. The surgery lasted six hours, followed by a two-week recovery period, according to Amy.
But Amy knew her son could handle it. Under Friedman’s care, Nick healed well, and the results soon became apparent.
Positive Outcomes with the Neurostimulator
With the treatment, Friedman says two key outcomes are hoped for: improved quality of life and reduced disease burden. Nick has experienced both.
“He’s definitely more alert during the day now and sleeping better,” Amy says. Nick recently graduated from high school and is attending Community Connections, a program that teaches life skills like cooking and grocery shopping. The improved sleep has made a noticeable difference in his participation. “It’s definitely made him more awake during school hours,” says Amy.
According to Friedman, Nick’s AHI is now less than 4. “We’re fortunate he’s on the green pathway. He’s got good adherence, and he’s also having a good response. It’s very positive,” he says.
Nick’s parents help him turn on the device each night, using a small handheld remote held to his chest. It’s programmed to activate 45 minutes after it’s turned on and run for 12 hours. If Nick wakes up sooner, the Lopezes turn the device off manually. Friedman says Nick’s average usage is around nine hours a night.
The neurostimulator, which Nick affectionately nicknamed “Iron Man,” provides Nick with better sleep and Amy with a much-needed sense of security. “He can toss and turn, and I don’t have to worry about anything getting twisted or caught or anything like that,” she says, adding that she’s sleeping better now, too.
Hope for Other Families
Amy hopes Nick’s story will inspire other families facing similar challenges. “I’d highly recommend [the treatment] for other kiddos in our situation,” she says. “Weigh the pros and the cons, and just see where your child fits into that situation.”
Since Nick received the device, the FDA approved it for children with Down syndrome down to 13 years old, have an AHI between 10 and 50, and cannot benefit from CPAP.
Amy says Nick’s future is bright. He stays active, playing football and basketball. He also drums, and Amy is now looking to get him into open mic nights. “We always joke he’s our little rock star,” she says.
Amy emphasizes that the treatment has dramatically enhanced Nick’s life—and stresses that Down syndrome doesn’t define who he is. “No matter what disability your child has, you can always override it to a certain degree,” she says. “With the right support and resources, your child can have an awesome, blessed life.”
Expanding Neurostimulator Access
Nick is among a handful of patients with Down syndrome who have received neurostimulator therapy at Colorado Children’s Hospital, says Friedman. Approximately 55% to 97% of people with Down syndrome have OSA.4
Since being approved for children with Down syndrome as young as age 13, Friedman says more families are inquiring about neurostimulators. Some children have been determined to not be good candidates following drug-induced sleep endoscopy. But the bigger challenge stopping some children from receiving the treatment, according to Friedman, is falling outside of the FDA-approved upper AHI limit.
“We have a few patients that are having over 100 events an hour, so for right now, we’re unable to implant them,” he says. “Those are the patients that are probably even more important to implant.”
Friedman hopes to see the AHI indications expanded.
A recently published case study showed that younger patients with Down syndrome could also benefit.3 The study reported on a 4-year-old boy with Down syndrome and OSA, Theodore “Theo” Scott of Knoxville, Tenn, who had been using CPAP since he was 1 year old. He received the implant in May 2023, and after one month, Scott’s sleep improved, and his AHI decreased by 40%.
Tim Herbert, chair, president, and CEO of Inspire Medical Systems, says the company is continually researching expanding indications for its neurostimulator. Last June, the device received FDA approval to increase the AHI from 65 to 100 events per hour and increase the warning for body mass index from 32 to 40 for adult patients.
“From the pediatrics side, we always look to see if we can help a younger age,” Herbert says. “There’s also a lot of pediatric patients who have obstructive sleep apnea, not necessarily just those with Down syndrome, so we look for other indications possibly to see if there are other groups of patients we can help.”
While Herbert doesn’t have the exact numbers on how many children with Down syndrome have received the implant due to patient privacy rights, Inspire has clinical studies on over 50 patients who have received the treatment whose data is being tracked.
Emerging Hypoglossal Nerve Stimulators
Inspire is the only FDA-approved hypoglossal nerve stimulator for OSA, though others are being tested, including LivaNova’s aura6000 and Nyxoah’s Genio. Both have been approved in Europe and received the CE mark in 2012 and 2019, respectively.
LivaNova announced last month that the company had concluded enrollment in the OSPREY trial to test the aura6000 for moderate to severe OSA treatment in the United States. In March, the company reported a positive predictive outcome in the trial’s success, meaning there’s a greater than 97.5% probability that the trial will meet its primary endpoint.
Nyxoah recently announced that the DREAM US study, designed to support marketing authorization in the United States, demonstrated a median 12-month AHI reduction of 70.8%, with similar AHI improvements in supine and non-supine sleeping positions.
Nyxoah is finalizing the fourth and final module submission in the premarket approval application to submit for FDA approval.
New Mexico Patient Benefits from Neurostimulation
Wyatt Varland, a 20-year-old from Albuquerque, NM, who has Down syndrome and autism spectrum disorder, also benefited from neurostimulation therapy for OSA.
Varland was diagnosed with OSA at age 5. He was prescribed CPAP before turning 14 after other health conditions worsened his OSA. However, CPAP did not provide adequate relief, leading to the prescription of bilevel positive airway pressure (BiPAP). Unfortunately, this too proved ineffective.
“He wasn’t getting the results he needed from the CPAP or BiPAP machines,” says his mother, Kim Varland, in a release. “The pressures were just so high. It was a lot of force.”
Wyatt’s OSA worsened over the years, resulting in hypercapnia—a condition characterized by excess carbon dioxide in the bloodstream. This heightened the family’s concerns due to Wyatt’s previous heart surgery.
The continuous disruptions took a toll on everyone’s sleep. “I feel like sleep apnea was a family disease,” Kim says in a release.
According to Kim, Wyatt’s doctors, Jeremy Prager, MD, and Norman Friedman, MD, at Children’s Hospital Colorado, suggested Inspire therapy as a solution. Still, Wyatt had to wait until he turned 18, as the treatment wasn’t yet approved for children with Down syndrome. His doctors determined he was a good candidate, and, in spring 2023, he received the implant.
Kim called the results “mind-blowing.” Wyatt went from barely sleeping to now sleeping over nine hours each night. His AHI dropped from 64 to under 10, improving his energy levels and stabilizing his cardiac issues, according to his parents.
“It’s the most amazing thing to see him with his angel face just sleeping soundly and breathing and not choking or gasping for air,” says Kim in a release, adding that she and her husband Dave can now sleep too.
References
- Dimopoulos K, Constantine A, Clift P, et al. Cardiovascular complications of Down syndrome: scoping review and expert consensus. Circulation. 2023;147(5):425-41.
- Tietjens JR, Claman D, Kezirian EJ, et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc. 2019;8(1):e010440.
- Wasserman I, Chieffe DJ, Gipson KS, et al. Hypoglossal nerve stimulation for obstructive sleep apnea in a young child with Down syndrome. Pediatrics. 2024;153(5):e2023063330.
- Hizal M, Satırer O, Polat SE, et al. Obstructive sleep apnea in children with Down syndrome: is it possible to predict severe apnea? Eur J Pediatr. 2022;181(2):735-43.
Photo caption: Nick Lopez
Photo credit: Lopez family


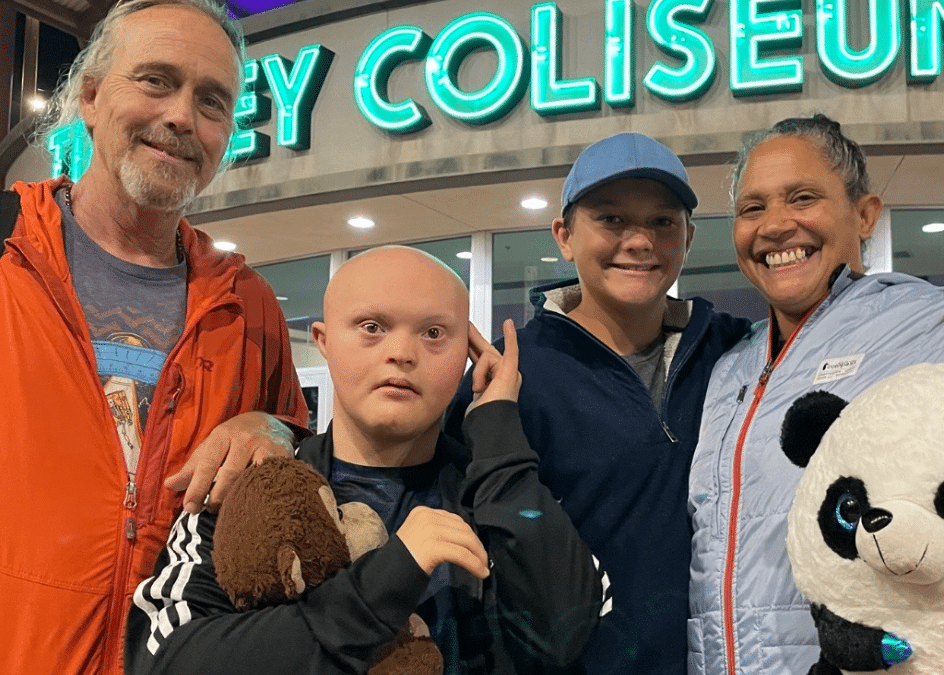




Leave a Reply