Summary: Patients with OSA who used CPAP therapy before total hip and knee replacement surgeries had significantly fewer complications post-surgery compared to those who didn’t use CPAP, finds a study conducted by the Hospital for Special Surgery. In a study presented by the … [Read more...]
OSA Impacts Patients’ Work, Relationships, and Mental Health
Summary: Apnimed Inc. conducted the SHINE (Sleep Health Inquiries on Needs and Emotions) survey, gathering insights from 1,500 people living with obstructive sleep apnea (OSA), which survey revealed the emotional, social, and behavioral impacts of OSA on daily life, including … [Read more...]
Vivos Granted Reimbursement Codes for Oral Appliances for OSA
Summary: Vivos Therapeutics Inc has announced that the American Medical Association (AMA) will introduce new Current Procedural Terminology (CPT) codes for Vivos CARE oral appliances, effective Jan 1, 2025. These codes are expected to facilitate insurance coverage and … [Read more...]
Incannex Provides Update on OSA Drug Candidate IHL-42X
Summary: Incannex Healthcare Inc provided an update on its lead drug candidate, IHL-42X, an oral therapy in development for the treatment of obstructive sleep apnea (OSA), highlighting progress in its phase 2/3 clinical trial. The clinical trial aims to evaluate IHL-42X in OSA … [Read more...]
Apnimed Completes Enrollment Early in Phase 3 Study for Oral OSA Treatment
Summary: Apnimed has completed enrollment for its phase 3 SynAIRgy study, which will evaluate the efficacy and safety of its lead candidate AD109, a potential first oral therapy for obstructive sleep apnea (OSA). The trial will focus on adults with mild, moderate, and severe OSA. … [Read more...]
Epilepsy Drug Slashes Breathing Pauses in OSA Patients
Summary: A clinical trial presented at the European Respiratory Society Congress in Vienna revealed that the epilepsy drug sulthiame significantly reduced symptoms of obstructive sleep apnea (OSA) in patients who cannot tolerate CPAP therapy. The study, conducted across multiple … [Read more...]
69% of OSA Patients Willing to Switch to Pill Treatment, Finds Quantum Research-Incannex Survey
Summary: A recent survey commissioned by Incannex reveals that 69% of OSA patients are open to switching from CPAP therapy to a pill-based treatment, with ease of use being the top reason. The study highlights mixed satisfaction with CPAP devices, as 61% of patients report weekly … [Read more...]
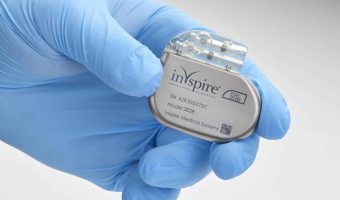
Long Beach Hospital Introduces Inspire for Children with Down Syndrome and OSA
Summary: MemorialCare Miller Children’s & Women’s Hospital Long Beach has completed its first two pediatric Inspire procedures for children with Down syndrome and obstructive sleep apnea (OSA). The FDA-approved Inspire device offers an alternative treatment for children who … [Read more...]
Virtual OSA Clinic Gem Sleep Unveils Upgraded Patient Portal
Summary: Gem Sleep, a virtual sleep apnea clinic, has launched an enhanced portal designed to make the treatment selection process more transparent and user-friendly for patients with obstructive sleep apnea (OSA). The updated portal includes features like a guided comparison … [Read more...]
XII Medical Raises $45M to Advance Neuromodulation Therapy for OSA
Summary: XII Medical Inc, a clinical-stage medical technology company focused on developing neuromodulation therapies for obstructive sleep apnea (OSA), has raised $45 million in Series B equity financing. The funding, led by Omega Funds with participation from new and existing … [Read more...]