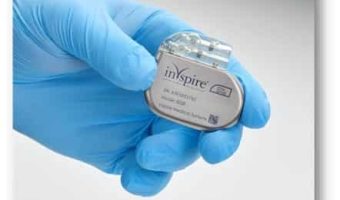
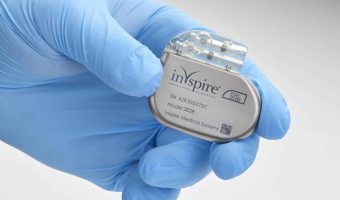
Summary: Inspire Medical Systems, a company specializing in minimally invasive solutions for obstructive sleep apnea, announced the approval of reimbursement for its Inspire therapy in France. The therapy, which received European Conformity Marking in 2010, has already secured … [Read more...]
Inspire Therapy Meets New EU Medical Device Standards
Summary: Inspire Medical Systems has achieved CE mark certification under the European Union’s updated Medical Device Regulation (EU MDR) for its sleep apnea treatment, Inspire therapy. This certification ensures compliance with the EU’s more stringent requirements, allowing … [Read more...]
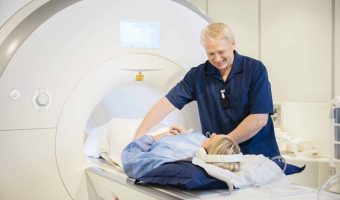
Inspire Gains EU Approval for Full-Body MRI Scans
Summary: Inspire Medical Systems has received approval under the European Union Medical Device Regulation (EU MDR) for full-body MRI scans for patients using its Inspire therapy for obstructive sleep apnea. This approval, which expands the previous scan limitations, applies … [Read more...]
Inspire Medical Implantable Pulse Generator Recall
By Sree Roy Sleep professionals are understandably anxious about product recall notices in the wake of the long-lasting negative impacts of the CPAP recall by Philips Respironics. But I predict that the recent recall of Inspire IV implantable pulse generators will ultimately be … [Read more...]
ECU Health Otolaryngologist Completes 100th Inspire Procedure
Summary: Brian Brodish, MD, an otolaryngologist with Eastern Carolina ENT, completed his 100th Inspire therapy procedure at ECU Health Medical Center on July 14. Inspire is an FDA-approved treatment for obstructive sleep apnea (OSA) designed for patients who cannot tolerate CPAP … [Read more...]
Inspire Recalls Component of Neurostimulator Therapy for OSA
Summary: Inspire Medical Systems has issued a recall for its Inspire IV implantable pulse generator used in treating obstructive sleep apnea due to a manufacturing defect. This defect may cause system malfunctions, including electrical leakage, potentially leading to serious … [Read more...]
WVU Medicine Celebrates 100 Inspire Implant Procedures
Summary: The WVU Medicine otolaryngology rest staff has accomplished its 100th Encourage implant process for obstructive snooze apnea (OSA). The plan, begun in 2017, has expanded beneath the leadership of Hassan Ramadan, MD, and his workforce, who emphasize the procedure’s … [Read more...]
Inspire Says Awareness is Growing for Its Sleep Apnea Therapy
Summary: Inspire Healthcare Techniques Inc reported a 28% 12 months-over-calendar year earnings advancement in Q1 2024, achieving $164 million, pushed by greater awareness of its Encourage treatment for obstructive slumber apnea. The business activated 66 new US facilities, … [Read more...]
4-Year-Old with Down Syndrome and OSA Benefits from Inspire
Summary: A 4-12 months-aged boy with Down syndrome and critical obstructive slumber apnea confirmed substantial advancement right after receiving a hypoglossal nerve stimulation implant, normally applied in older people and also authorised for adolescents with Down syndrome and … [Read more...]