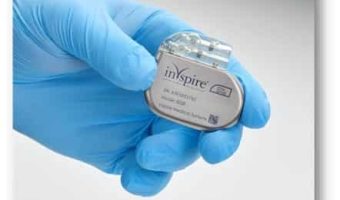
Summary: Inspire Medical Systems has achieved CE mark certification under the European Union’s updated Medical Device Regulation (EU MDR) for its sleep apnea treatment, Inspire therapy. This certification ensures compliance with the EU’s more stringent requirements, allowing … [Read more...]
Natus Appoints Veteran Medical Device Leader as New CEO
Summary: Natus has appointed Chris Landon as its new CEO. Landon brings over two decades of experience in the medical device industry, focusing on image-guided therapy devices, cardiology, and neurodiagnostics. He previously held executive roles at Philips and Medtronic and … [Read more...]
Sunglasses-Like Device Promises Better Sleep Via Blue Light Sleep Review
Summary: AYO, specializing in circadian light therapy, introduced its wearable device at SLEEP 2024. The device, worn like sunglasses, uses blue-turquoise light to help regulate circadian rhythms, aiming to improve sleep quality, mood, and overall wellness. AYO’s technology, … [Read more...]
Radiofrequency Device Offers Lasting Relief for Chronic Rhinitis
Summary: Neurent Medical announced the publication of a 12-month study in Laryngoscope Investigative Otolaryngology demonstrating that its Neuromark system, a radiofrequency ablation device, improves symptoms and quality of life for chronic rhinitis patients. The CLARITY study … [Read more...]
FDA Said It Never Inspected Dental Lab That Made Controversial AGGA Device
By Brett Kelman and Anna Werner, CBS News May 13, 2024 The FDA never inspected Johns Dental Laboratories during more than a decade in which it made the Anterior Growth Guidance Appliance, or “AGGA,” a dental device that has allegedly harmed patients and is now the subject of a … [Read more...]
Device Uses Gamma Sound and Light Therapy to Enhance Sleep | Sleep Review
Summary: Adaptive Seem Technologies Inc has released Sound+Treatment, a system that uses 40 Hz gamma seem and light-weight treatment to market much better rest. It replicates historical sound therapy tactics with proprietary conquer appears to help peace and boost slumber good … [Read more...]
Wearable Device Offers Stress and Sleep Hormone Monitoring
Summary: A new wearable device, launching this summer, leverages proprietary sensor technology for non-invasive, continuous monitoring of cortisol and melatonin levels, providing real-time insights into stress and sleep. Originating from research on inflammation markers in … [Read more...]
Oral Device Maker ProSomnus Reports Record Revenue for 2023
Summary: ProSomnus Inc, specializing in oral appliance remedy for obstructive slumber apnea, claimed a 43% enhance in 2023 income to $27.7 million, marking its eighth consecutive quarter of history earnings. Development was attributed to the adoption of its EVO item, elevated … [Read more...]