Summary: Vivos Therapeutics reported a 17% increase in Q3 2024 revenue and a 27% reduction in operating loss, driven by its focus on cost-cutting and strategic milestones, including FDA 510(k) clearance for its oral device to treat pediatric sleep apnea. The company also received … [Read more...]
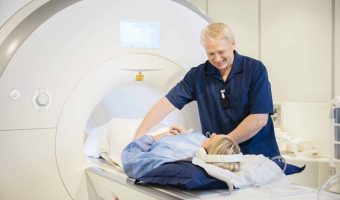
Inspire Gains EU Approval for Full-Body MRI Scans
Summary: Inspire Medical Systems has received approval under the European Union Medical Device Regulation (EU MDR) for full-body MRI scans for patients using its Inspire therapy for obstructive sleep apnea. This approval, which expands the previous scan limitations, applies … [Read more...]
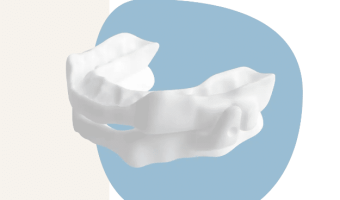
Australian-Designed Oral Appliance Gains FDA Clearance
Summary: GoodSleepCo, an Australian medical device company, has received FDA 510(k) clearance for its hushd Pro Avera oral appliance, which treats snoring and mild to moderate obstructive sleep apnea. Custom-made splints reposition the lower jaw to prevent airway blockage during … [Read more...]
Vivos Gains After FDA Clears Oral Appliances for Severe OSA
Summary: According to the Vivos Therapeutics fourth-quarter and total-year 2023 report, there has been beneficial momentum considering the fact that the Food and drug administration clearance of its Treatment oral appliances for severe OSA in grownups. This momentum is evidenced … [Read more...]